Emerson Stokes Research Group Archive
Research Papers
Polyhedron 2025
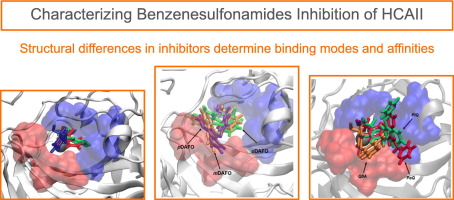
Mahshid Attarroshan and Alex Cutright contributed to this Polyhedron paper published by the Steve Gwaltney group at MSU. [Click on the image above to link to the paper.] Congrats to Loan, Mahshid, and all who contributed!
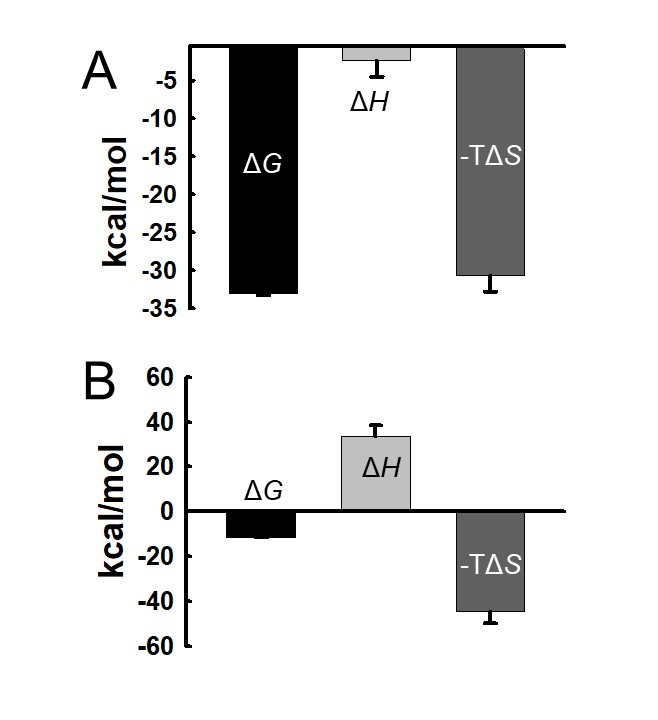
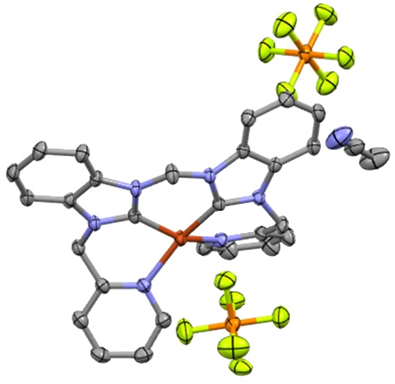
Nguyen, LTT ; Attarroshan, M; Cutright, AJ; Stokes, SL; Emerson, JP; Gwaltney, SR; Understanding binding behavior of human carbonic anhydrase II with aromatic benzenesulfonamides by molecular dynamics simulations and biophysical characterization. Polyhedron, 2025, 280, 117683. https://doi.org/10.1016/j.poly.2025.117683
Nguyen, LTT ; Attarroshan, M; Cutright, AJ; Stokes, SL; Emerson, JP; Gwaltney, SR; Understanding binding behavior of human carbonic anhydrase II with aromatic benzenesulfonamides by molecular dynamics simulations and biophysical characterization. Polyhedron, 2025, 280, 117683. https://doi.org/10.1016/j.poly.2025.117683
Tetrahedron Lett 2025
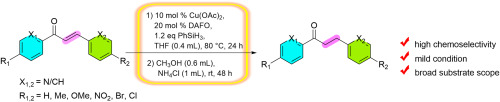
Mahshid Attarroshan's paper the reduction of chalcones was recently reported in Tetrahedron Lett. [Click on the image above to link to the paper.] Congrats to Mahshid and all who contributed!
Attarroshan, M; Vazquez, BC; Andrews, CR; Stokes, SL; Emerson, JP; Selective Copper-Catalyzed 1,4-Reduction of α,β-Unsaturated Ketones Using Phenylsilane. Tetrahedron Lett, 2025, 161, 155567. https://doi.org/10.1016/j.tetlet.2025.155567.
Attarroshan, M; Vazquez, BC; Andrews, CR; Stokes, SL; Emerson, JP; Selective Copper-Catalyzed 1,4-Reduction of α,β-Unsaturated Ketones Using Phenylsilane. Tetrahedron Lett, 2025, 161, 155567. https://doi.org/10.1016/j.tetlet.2025.155567.
Tetrahedron Lett 2025
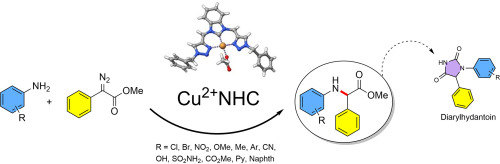
Mohsen Teimouri's reporting two new Cu-NHC complexes with triazol donor groups was reported as part of a special issue in Tetrahedron Lett. This invited paper showcases new options for pincer ligands and sets-the-stage for some exciting on-going work in the ESRG! [Click on the image above to link to the paper.] Congrats to Mohsen and all who contributed!
Teimouri, M; Attarroshan, M; Donnadieu, B; Stokes, SL; Emerson, JP; Copper(II) NHC pincer complexes for carbene insertion into the N—H bond of aniline. Tetrahedron Letters (invited article), 2025, 162, 155588. https://doi.org/10.1016/j.tetlet.2025.155588
Teimouri, M; Attarroshan, M; Donnadieu, B; Stokes, SL; Emerson, JP; Copper(II) NHC pincer complexes for carbene insertion into the N—H bond of aniline. Tetrahedron Letters (invited article), 2025, 162, 155588. https://doi.org/10.1016/j.tetlet.2025.155588
Molecules 2024
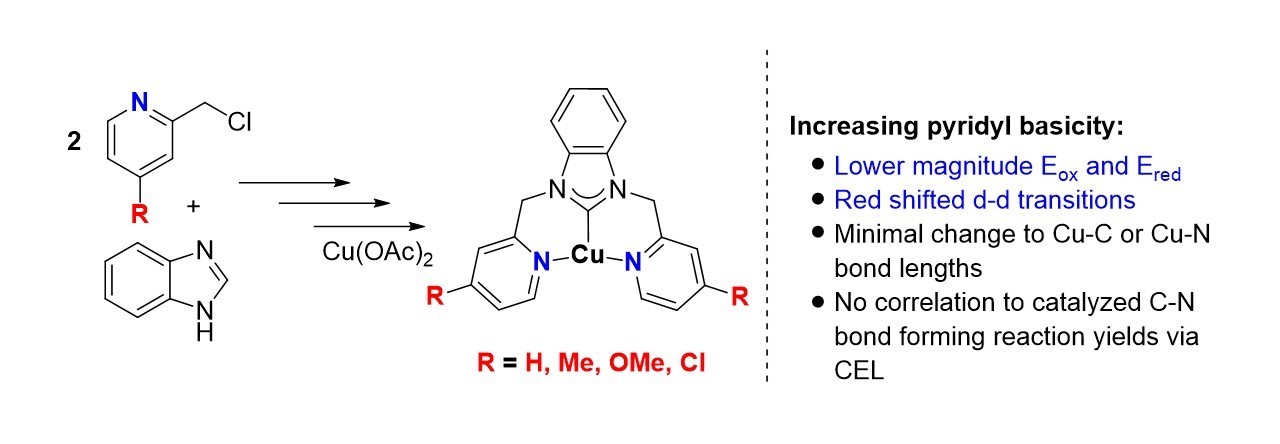
Bhupendra Adhikari's paper reporting a series of tridentate NHC copper(II) complexes with pyridinyl substitutions was published in MDPI's journal Moleulces. This paper the impact of inductive and resonance effects on pyridinyl ligands in pincer compounds. We also explored how these changes impact CEL reactions. [Click on the image above to link to the paper.] Congrats to Bhupen and all who contributed!
Adhikari, B; Raju, S; Femi Awoyemi, R; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Synthesis and Synthesis and Characterization of Symmetrical N-Heterocyclic Carbene Copper(II) Complexes — An Investigation of the Influence of Pyridinyl Substituents. Molecules, 2024, 29(15) 3542. Doi: 10.3390/molecules29153542
Adhikari, B; Raju, S; Femi Awoyemi, R; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Synthesis and Synthesis and Characterization of Symmetrical N-Heterocyclic Carbene Copper(II) Complexes — An Investigation of the Influence of Pyridinyl Substituents. Molecules, 2024, 29(15) 3542. Doi: 10.3390/molecules29153542
Molecules 2024
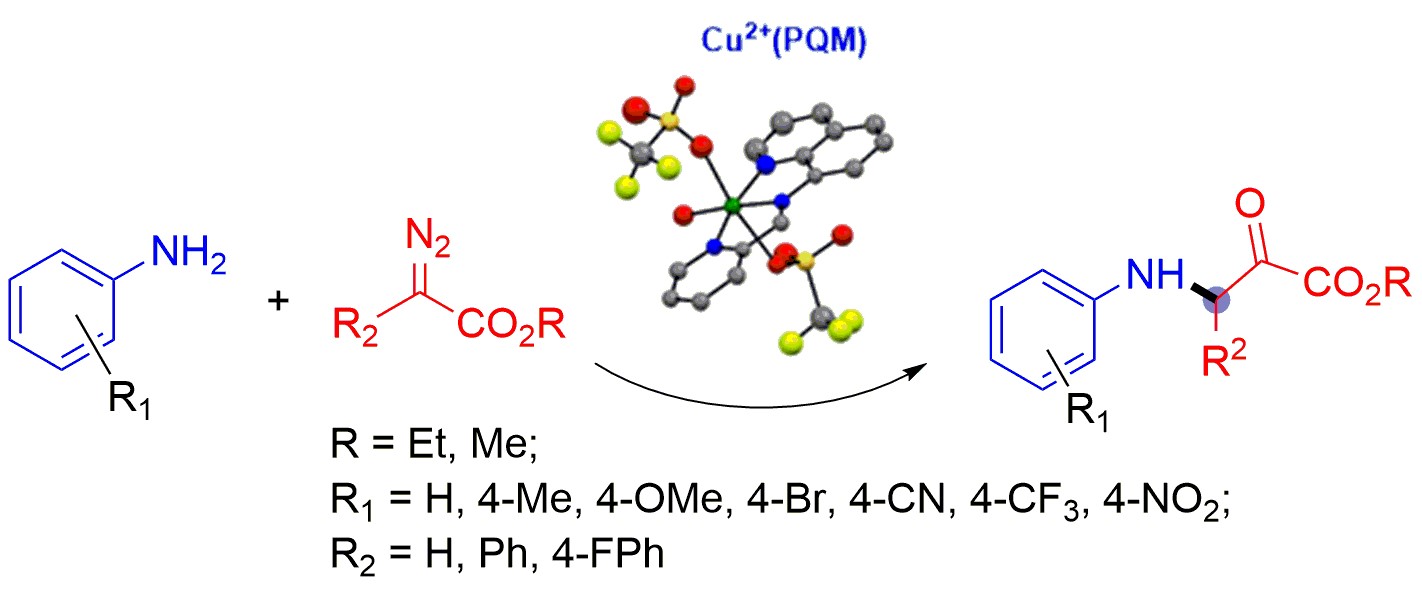
Mohsen Teimouri's paper showcasing the PQM ligand was reported in Molecules. This paper showcases diazo complex activation and new C-C bond foring reactions with good yields. [Click on the image above to link to the paper.] Congrats to Mohsen and all who contributed!
Teimouri, M; Raju, S; Schmittou, A; Pierce, BS; Awoyemi, RF; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Aminoquinoline-based Tridentate (NNN)-Copper Catalyst towards Redox-Neutral Cross-coupling of Anilines with Diazo compounds. Molecules 2024, 29(3), 730. doi: 10.3390/molecules29030730.
Teimouri, M; Raju, S; Schmittou, A; Pierce, BS; Awoyemi, RF; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Aminoquinoline-based Tridentate (NNN)-Copper Catalyst towards Redox-Neutral Cross-coupling of Anilines with Diazo compounds. Molecules 2024, 29(3), 730. doi: 10.3390/molecules29030730.
Dalton Trans 2024
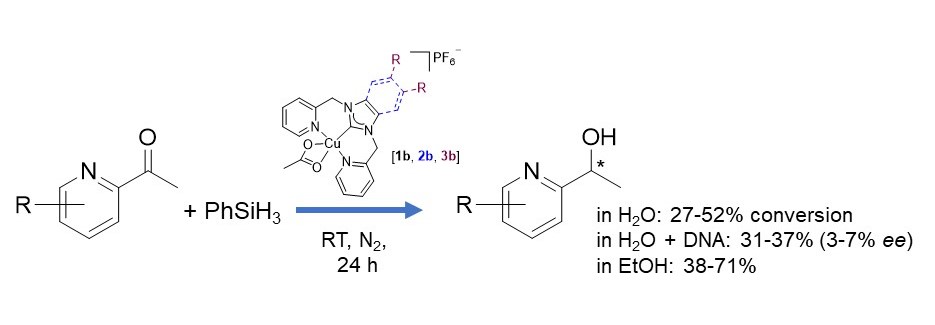
Dr. M. Sharma's paper on ketone reduction by PhSiH3 catalyzed by a tridentate NHC copper(II) complex was published in Dalton Transactions. This reaction worked reasonably well in water, but did not support enantioselectivity when complexed with DNA. [Click on the image above to link to the paper.] Congrats to Mitu and all who contributed!
Sharma, M; Perkins, AM; Awoyemi, RF; Raju, S; Schmittou, A; Pierce, BS; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Steps towards enantioselective ketone reduction with silanes catalyzed by DNA-copper(II) N-heterocyclic carbene-based hybrid catalysts via copper hydride formation, Dalton Transactions 2024, 53(7), 3180-3190. doi: 10.1039/d3dt03406b.
Sharma, M; Perkins, AM; Awoyemi, RF; Raju, S; Schmittou, A; Pierce, BS; Donnadieu, B; Wipf, DO; Stokes, SL; Emerson, JP; Steps towards enantioselective ketone reduction with silanes catalyzed by DNA-copper(II) N-heterocyclic carbene-based hybrid catalysts via copper hydride formation, Dalton Transactions 2024, 53(7), 3180-3190. doi: 10.1039/d3dt03406b.
Dalton Trans 2023
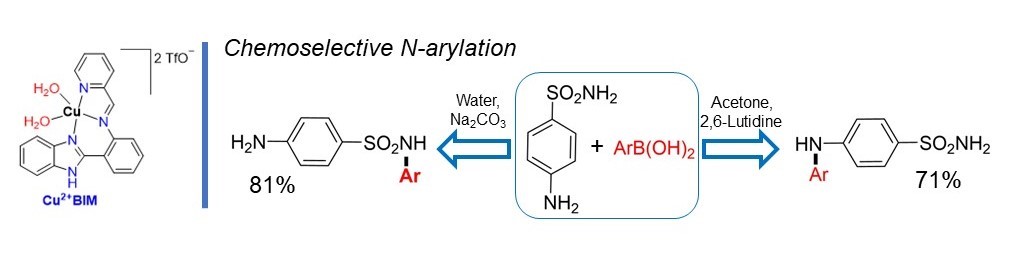
Dr. Raju's paper on the chemoselectivity of Cu-catalyzed CEL reactions toward sulfonilamide in Dalton Transactions. This paper showcases how reaction conditions dramatically impact the favored pathway of a reaction. [Click on the image above to link to the paper.] Congrats to Selvam and all who contributed!
Raju, S; Teimouri, T; Donnadieu, B; Stokes, SL; Emerson, JP; Synthesis, Structure, and properties tridentate Copper(II) complexes for the chemoselective N-arylation of sulfanilamide via Chan-Evens-Lam cross-coupling, Dalton Transactions 2023, 52(43), 15986-15994. doi: 10.1039/d3dt02659k. (Impact factor: 4.57, Times cited: 0)
Raju, S; Teimouri, T; Donnadieu, B; Stokes, SL; Emerson, JP; Synthesis, Structure, and properties tridentate Copper(II) complexes for the chemoselective N-arylation of sulfanilamide via Chan-Evens-Lam cross-coupling, Dalton Transactions 2023, 52(43), 15986-15994. doi: 10.1039/d3dt02659k. (Impact factor: 4.57, Times cited: 0)
Asian J Org Chem 2023
In a collaborative effort between L Mohan, JH Kim, and the Emerson/Stokes group, we reported a new method for coupling reactions between enamines and alkynes in AJOC. [Click on the image above to link to the paper.] Congrats to Lakavathu Mohan and all who contributed!
Mohan, L; Lee, JE; Srinivasarao, M.; Cutright, AJ; Raju, S; Stokes, SL; Emerson, JP; Kim, JH; Cu‒Catalyzed Cross-Coupling reactions of Enamines and Terminal Alkynes: Access of Propargylamines as Neurodegenerative Disorder Agents, Asian Journal of Organic Chemistry 2023, 12(10), e202300375. doi: 10.1002/ajoc.202300375
Mohan, L; Lee, JE; Srinivasarao, M.; Cutright, AJ; Raju, S; Stokes, SL; Emerson, JP; Kim, JH; Cu‒Catalyzed Cross-Coupling reactions of Enamines and Terminal Alkynes: Access of Propargylamines as Neurodegenerative Disorder Agents, Asian Journal of Organic Chemistry 2023, 12(10), e202300375. doi: 10.1002/ajoc.202300375
Eur J Org Chem 2023
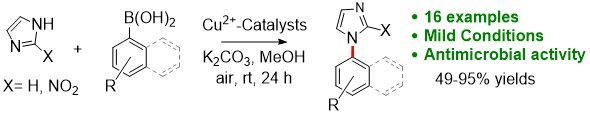
Bhupendra Akhikari's paper on copper(II) catalyzed CEL reactions was published in EJOC. This paper showcases an effective method for C-N bond forming reactions with an in situ generated Cu-NHC complex. [Click on the image above to link to the paper.] Congrats to Bhupen and all who contributed!
Adhikari, B; Teimouri, M; Akin, JW; Raju, S; Stokes, SL; Emerson, JP; Cu2+NHC-Complex for Chan-Evans-Lam Cross-Coupling Reactions of N-Heterocyclic Compounds and Arylboronic Acids, European Journal of Organic Chemistry 2023, e202300620. Doi: 10.1002/ejoc.202300620
Adhikari, B; Teimouri, M; Akin, JW; Raju, S; Stokes, SL; Emerson, JP; Cu2+NHC-Complex for Chan-Evans-Lam Cross-Coupling Reactions of N-Heterocyclic Compounds and Arylboronic Acids, European Journal of Organic Chemistry 2023, e202300620. Doi: 10.1002/ejoc.202300620
J Inorg Biochem 2023
Alex Cutright's paper on zinc(II) and DNA binding to AdcR was published in J. Inorg. Biochem. This paper showcases the key thermodynamics that govern zinc(II) homeostasis in S. pneumoniae. [Click on the image above to link to the paper.] Congrats to Alex and all who contributed!
Cutright, AJ; Matthews, EL; Aulds, JM; Al Mohanna, T; Thornton, JA; Stokes, SL; Emerson, JP; Calorimetric analysis of AdcR and its interactions with zinc(II) and DNA, Journal of Inorganic Biochemistry 2023, 247:112305. doi: 10.1016/j.jinorgbio.2023.112305 (Impact factor: 4.336, Times cited: 0)
Cutright, AJ; Matthews, EL; Aulds, JM; Al Mohanna, T; Thornton, JA; Stokes, SL; Emerson, JP; Calorimetric analysis of AdcR and its interactions with zinc(II) and DNA, Journal of Inorganic Biochemistry 2023, 247:112305. doi: 10.1016/j.jinorgbio.2023.112305 (Impact factor: 4.336, Times cited: 0)
Tetrahedron Lett 2023
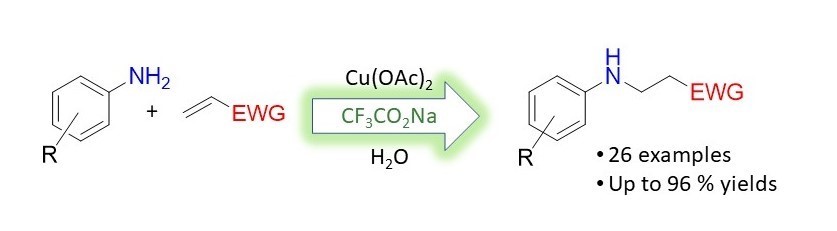
Erfan Masaeli's paper focusing on aza Micheal additions was recently reported in Tet. Lett! [Click on the image above to link to the paper.] Congrats to Erfan and all who contributed!
S. Erfan Masaeli, Mohsen Teimouri, Bhupendra Adhikari, Mahshid Attarroshan, James W. Akin, Selvam Raju, Sean L. Stokes, Joseph P. Emerson, Sodium Trifluoroacetate mediated Copper-Catalyzed aza-Michael addition of α,β-unsaturated olefins with aromatic amines, Tetrahedron Letters 2023, 122. 154520. Doi: 10.1016/j.tetlet.2023.154520.
S. Erfan Masaeli, Mohsen Teimouri, Bhupendra Adhikari, Mahshid Attarroshan, James W. Akin, Selvam Raju, Sean L. Stokes, Joseph P. Emerson, Sodium Trifluoroacetate mediated Copper-Catalyzed aza-Michael addition of α,β-unsaturated olefins with aromatic amines, Tetrahedron Letters 2023, 122. 154520. Doi: 10.1016/j.tetlet.2023.154520.
Inorganics 2023
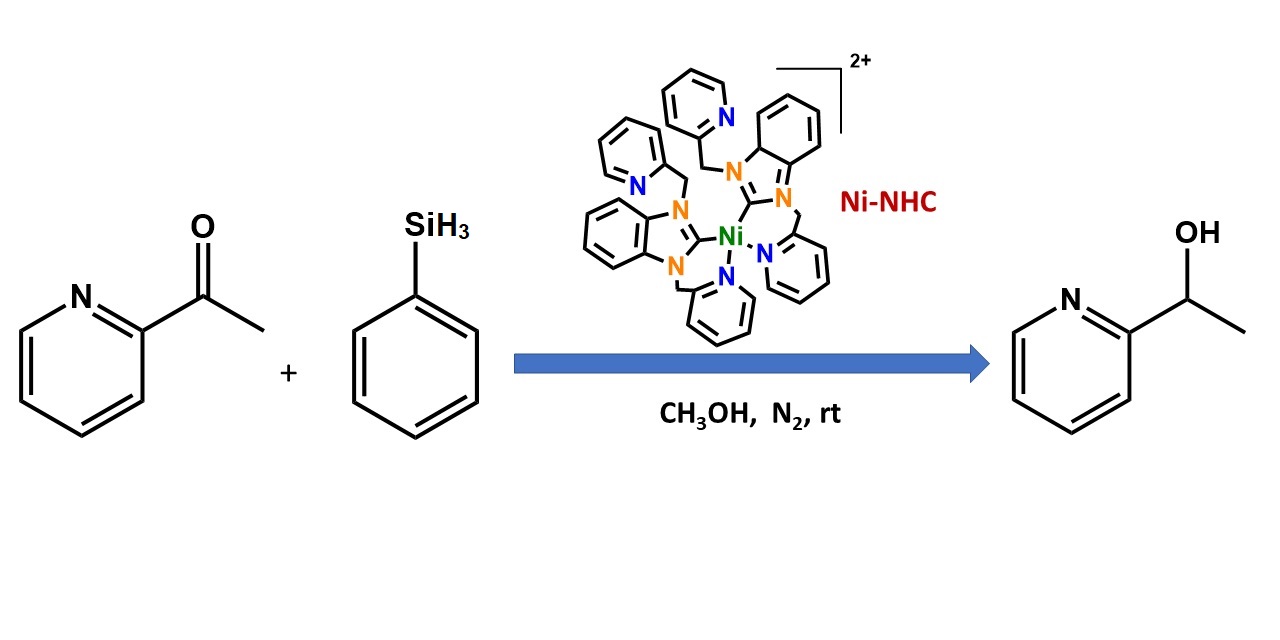
Dr. M. Sharma's paper on ketone reduction by phenylsilane catalyzed by a tetradentate NHC copper(II) complexes was published in MDPI's journal Inorganics. [Click on the image above to link to the paper.] Congrats to Mitu and all who contributed!
Mitu Sharma, Amanda M. Perkins, Alison K. Duckworth, Emily J. Rouse, Bruno Donnadieu, Bhupendra Adhikari, Sean L. Stokes, and Joseph P. Emerson,* Nickel(II) N-heterocyclic carbene complex as catalyst for the hydrogenation of 2-acetylpyridine under mild conditions, Inorganics 2023, 11, 120. Doi: 10.3390/inorganics11030120
Mitu Sharma, Amanda M. Perkins, Alison K. Duckworth, Emily J. Rouse, Bruno Donnadieu, Bhupendra Adhikari, Sean L. Stokes, and Joseph P. Emerson,* Nickel(II) N-heterocyclic carbene complex as catalyst for the hydrogenation of 2-acetylpyridine under mild conditions, Inorganics 2023, 11, 120. Doi: 10.3390/inorganics11030120
Archieves of Biochemsitry and Biophysics 2022
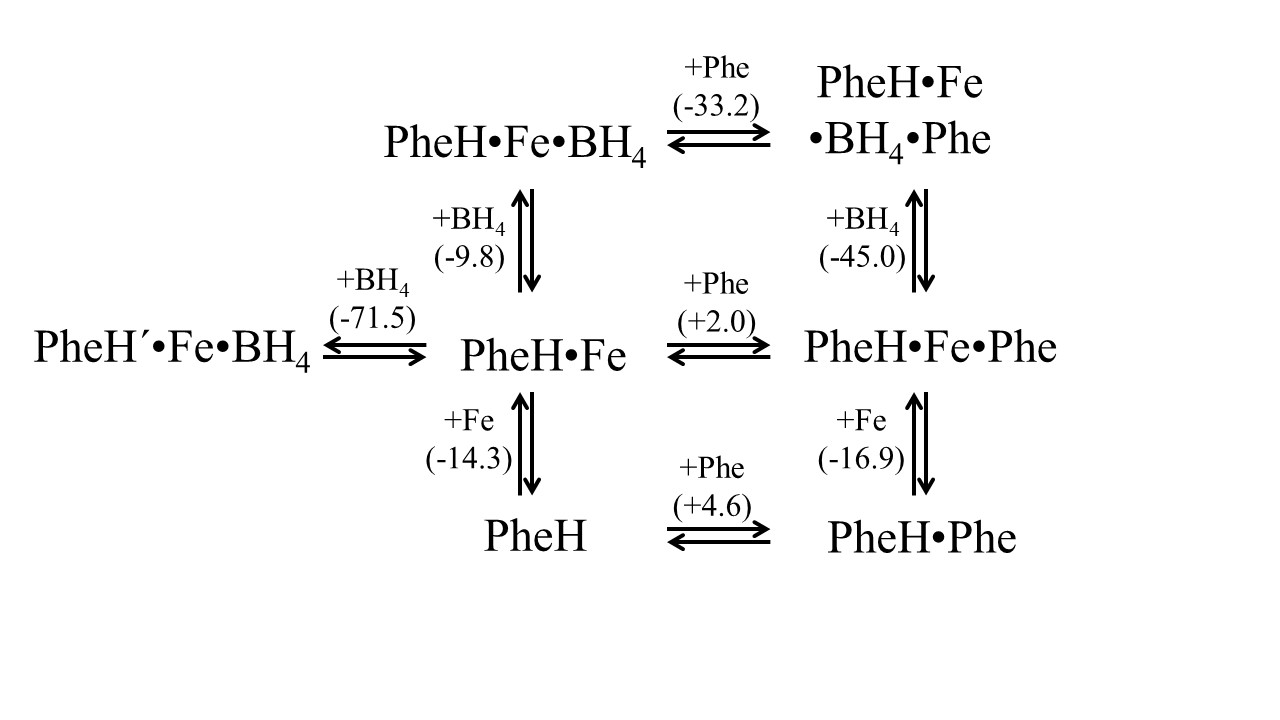
Dr. Mingjie Li's paper on substate and cofactor binding to PheH was published in collaboration with Paul Fitzpatrick in ABB. This paper adds to our long interests in understanding the pre-catalytic orginization of non-heme iron proteins. [Click on the image above to link to the paper.] Congrats to Mingjie and all who contributed!
Mingjie Li, Bishnu P. Subedi, Paul F. Fitzpatrick,* Joseph P. Emerson, Thermodynamics of iron, tetrahydrobiopterin, and phenylalanine binding to phenylalanine hydroxylase from Chromobacterium violaceum, Archives of Biochemistry and Biophysics, 2022, 729, 109378. Doi: 10.1016/j.abb.2022.109378.
Mingjie Li, Bishnu P. Subedi, Paul F. Fitzpatrick,* Joseph P. Emerson, Thermodynamics of iron, tetrahydrobiopterin, and phenylalanine binding to phenylalanine hydroxylase from Chromobacterium violaceum, Archives of Biochemistry and Biophysics, 2022, 729, 109378. Doi: 10.1016/j.abb.2022.109378.
Chemistry 2022
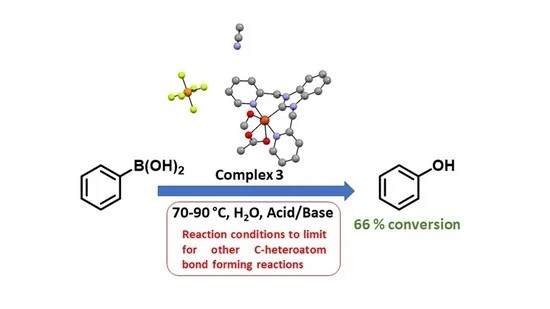
Dr. M. Sharma's paper on boronic acid activation by tridentate NHC copper(II) complexes was published in MDPI's journal Chemistry. This invited paper showcases optimized conditions for the conversion of boronic acids to phenols. This work also highlights conditions that do not favor this process when targeting other C-C or C-N bond forming reactions. [Click on the image above to link to the paper.] Congrats to Mitu and all who contributed!
Mitu Sharma, Bhupendra Adhikari, Raymond Femi, Amanda M. Perkins, Alison K. Duckworth, Bruno Donnadieu David O. Wipf, Sean L. Stokes, Joseph P. Emerson,Copper(II) NHC catalyst for the formation of phenol from arylboronic acids, Chemistry 2022, 4(2), 560-575; https://doi.org/10.3390/chemistry4020040
Mitu Sharma, Bhupendra Adhikari, Raymond Femi, Amanda M. Perkins, Alison K. Duckworth, Bruno Donnadieu David O. Wipf, Sean L. Stokes, Joseph P. Emerson,Copper(II) NHC catalyst for the formation of phenol from arylboronic acids, Chemistry 2022, 4(2), 560-575; https://doi.org/10.3390/chemistry4020040
Chem Biodivers 2022
Dr. Raju's paper exploring a library of substituted nitroimidazole toward anti S. pneumoniae activity. This work also highlights new copper dependent cross-coupling agents developed in the lab. [Click on the image above to link to the paper.] Congrats to Selvam and all who contributed!
Selvam Raju, Patrick E. Sheridan, Alanna K. Hauer, Allyn E. Garrett, Danielle E. McConnell, Justin A. Thornton, Sean L. Stokes,* and Joseph P. Emerson,* Cu-Catalyzed Chan-Evans-Lam Coupling reactions of 2-Nitroimidazole with Aryl boronic acids: An effort toward new bioactive agents against S. pneumoniae. Chemistry & Biodiversity 2022, 19(8):e202200327. Doi: 10.1002/cbdv.202200327
Selvam Raju, Patrick E. Sheridan, Alanna K. Hauer, Allyn E. Garrett, Danielle E. McConnell, Justin A. Thornton, Sean L. Stokes,* and Joseph P. Emerson,* Cu-Catalyzed Chan-Evans-Lam Coupling reactions of 2-Nitroimidazole with Aryl boronic acids: An effort toward new bioactive agents against S. pneumoniae. Chemistry & Biodiversity 2022, 19(8):e202200327. Doi: 10.1002/cbdv.202200327
Inorg Chem 2022
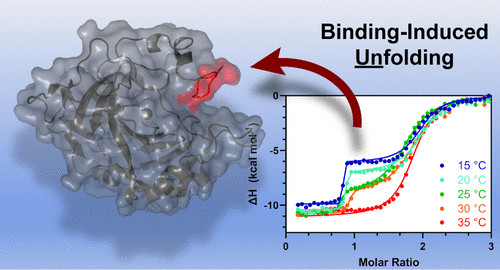
K. McConnell's paper correlating metal ion coordination to protein orginization was published in Inorganic Chemistry! This collaborative effort between the ESRG and Nick Fitzkee's group showcases how metal ion coordination can lead to protein orginization and apprently in some cases it can lead to disorginization too. [Click on the image above to link to the paper.] Congrats Kayla!
Kayla D. McConnell, Nicholas C. Fitzkee, Joseph P. Emerson, Metal Ion Binding Can Induce Local Folding and Unfolding in Human Carbonic Anhydrase II, Inorganic Chemistry 2022, 61 1249-1253. Doi:10.1021/acs.inorgchem.1c03271
Kayla D. McConnell, Nicholas C. Fitzkee, Joseph P. Emerson, Metal Ion Binding Can Induce Local Folding and Unfolding in Human Carbonic Anhydrase II, Inorganic Chemistry 2022, 61 1249-1253. Doi:10.1021/acs.inorgchem.1c03271
Cat Comm 2021
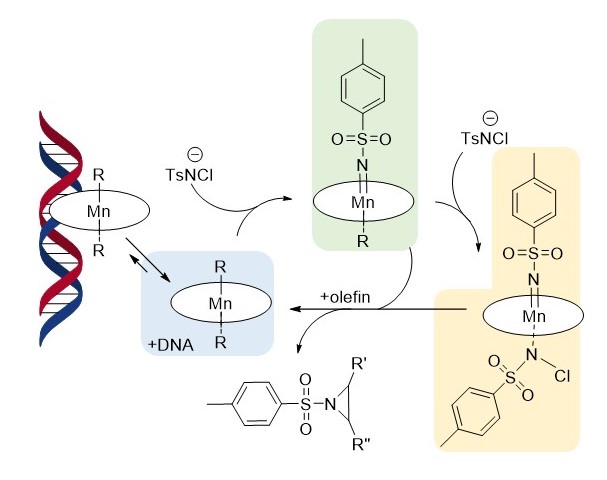
D. Wolgemuth's paper on olefin aziridination in water using a Mn-TmPyP4 catalyst was published! This marks the group's first effort to use water sobuble porphyrin complexes to conduct organic transformations. [Click on the image above to link to the paper.] Congrats to Danny and all who contributed!
Daniel K. Wolgemuth, Sydnee D. Elmore, James D. Cope, Patrick E. Sheridan, Sean L. Stokes, Joseph P. Emerson, Manganese-Catalyzed Aziridination of Olefins with Chloramine-T in Water and Buffered Aqueous Solutions. Catalysis Communications 2021, 150 106275. Doi 10.1016/j.catcom.2020.106275
Daniel K. Wolgemuth, Sydnee D. Elmore, James D. Cope, Patrick E. Sheridan, Sean L. Stokes, Joseph P. Emerson, Manganese-Catalyzed Aziridination of Olefins with Chloramine-T in Water and Buffered Aqueous Solutions. Catalysis Communications 2021, 150 106275. Doi 10.1016/j.catcom.2020.106275
Organometallics 2020
J. Cope's paper characterizing our first tetradentate copper(II) N-heterocyclic carbene complex was published in Organometallics! This marks the group's first new copper(II) complex that shows good CEL chemistry. [Click on the image above to link to the paper.] Congrats to James and all who contributed!
James D. Cope, Patrick E. Sheridan, Christopher J. Galloway, Raymond Femi Awoyemi, Sean L. Stokes, Joseph P. Emerson, Synthesis and characterization of a tetradentate, N-heterocyclic carbene copper(II) complex and its use as a Chan-Evans-Lam coupling catalyst. Organometallics 2020, 39 (24) 4457-4464. Doi: 10.1021/acs.organomet.0c00552
James D. Cope, Patrick E. Sheridan, Christopher J. Galloway, Raymond Femi Awoyemi, Sean L. Stokes, Joseph P. Emerson, Synthesis and characterization of a tetradentate, N-heterocyclic carbene copper(II) complex and its use as a Chan-Evans-Lam coupling catalyst. Organometallics 2020, 39 (24) 4457-4464. Doi: 10.1021/acs.organomet.0c00552
Earlier work from the ESRG
Streptococcus pneumoniae metal homeostasis alters cellular metabolism.
Lindsey R. Burcham, Rebecca A. Hill, Rachel C. Caulkins, Joseph P. Emerson, Bindu Nanduri, Jason W. Rosch, Nicholas C. Fitzkee, Justin A. Thornton, Metallomics, 2020, 12, 1416-1427. doi: 10.1039/D0MT00118J
Tuning the Copper(II)/Copper(I) Redox Potential for More Robust Copper-Catalyzed C–N Bond Forming Reaction.
James D. Cope, Henry U. Valle, Ruby S. Hall, Ekta Goel, S. Biswas, Michael P. Hendrich, David O. Wipf, Sean L. Stokes, Joseph P. Emerson, European Journal of Inorganic Chemistry 2020; 2020(14):1278-1285. doi: 10.1002/ejic.201901269.
Thermodynamics of iron(II) and substrate binding to the ethylene-forming enzyme.
Mingjie Li, Salette Martinez, Robert P. Hausinger, Joseph P. Emerson, Biochemistry 2018; 57:5696-5705. doi: 10.1021/acs.biochem.8b00730.
Synthesis, Characterization, and Structure of a [(phen)2Cu(OTf)]OTf Complex; An Efficient Nitrogen Transfer Pre-catalyst.
Henry U. Valle, Kathleen M. Riley, Dylan E. Russell, Daniel K. Wolgemuth, Shanterell L. Redd, Sean L. Stokes, Joseph P.
Emerson, ChemistrySelect 2018; 22:1123-1135. doi: 10.1002/slct.201800588
The Irving William series and the 2-His-1-carboxylate facial triad: A thermodynamic study of Mn2+, Fe2+, and Co2+ binding to taurine/α-ketoglutarate dioxygenase (TauD).
Mingjie Li, Kate L. Henderson, Salette Martinez, Robert P. Hausinger, Joseph P. Emerson, Journal of Biological Inorganic
Chemistry, 2018; doi: 10.1007/s00775-018-1574-4.
Resolving Distinct Molecular Origins for Copper Effects on PAI-1
Joel C. Bucci, Carlee S. McClintock, Yuzhuo Chu, Gregory L. Ware, Kayla D. McConnell, Joseph P. Emerson, Cynthia B.
Peterson, Journal of Biological Inorganic Chemistry, 2017; 22:1123-1135. doi:10.1007/s00775-017-1489-5
Global Stability of an α-Ketoglutarate-Dependent Dioxygenase (TauD) Using Differential Scanning Calorimetry
Kate L. Henderson, Mingjie Li, Salette Martinez, Robert P. Hausinger, Joseph P. Emerson, Biochimica Biophysica Acta 2017; 1851-994. doi: 10.1016/j.bbagen.2017.02.018
ITC Methods for Assessing Buffer/Protein Interactions using Steady-State Kinetics: A reactivity study of Homoprotocatechuate 2,3-Dioxygenase
Kate L. Henderson, Delta K. Boyles, Vu H. Le, Edwin A. Lewis, and Joseph P. Emerson, Methods in Enzymology 2016; 567; 257-78. doi: 10.1016/bs.mie.2015.08.034
Calorimetric and spectroscopic investigations of the binding of metallated porphyrins to G-quadruplex DNA
Jesse I. DuPont, Kate L. Henderson, Amanda Metz, Vu H. Le, Joseph P. Emerson, Edwin A. Lewis, Biochimica Biophysica Acta 2015 1860(5):902-9. doi: 10.1016/j.bbagen.2015.09.004.
Thermodynamics of Substrate Binding to the Metal Site in Homoprotocatechuate 2,3-Dioxygenase: Using ITC under anaerobic conditions to study enzyme-substrate interactions
Kate L. Henderson, Danielle H. Francis, Edwin A. Lewis, Joseph P. Emerson, Biochimica Biophysica Acta, 2015 1860(5):910-6. doi: 10.1016/j.bbagen.2015.07.013
Characterization of the Copper(II) Binding Sites in Human Carbonic Anhydrase II.
Whitnee L. Nettles, He Song, Nicholas C. Fitzkee, Joseph P. Emerson, Inorganic Chemistry, 2015 54(12), 2278-2283. DOI: 10.1021/acs.inorgchem.5b00057
Iodide-Induced Organothiol Desorption and Photochemical Reaction, Gold Nanoparticle (AuNP) Fusion, and SERS Signal
Reduction in Organothiol-Containing AuNP Aggregates
Ganganath S. Perera, Allen LaCour, Yadong Zhou, Kate L. Henderson, Shengli Zou, Felio Perez, Joseph P. Emerson, Dongmao Zhang, Journal of Physical Chemistry C, 2015 1119(8), 4261-4267. DOI: 10.1021/jp512168z
Calorimetric Assessment of Fe2+ Binding to α-Ketoglutarate/Taurine Dioxygenase: Ironing Out the Energetics of Metal
Coordination by the 2-His-1-Carboxylate Facial Triad
Kate L. Henderson, Tina Müller, Robert Hausinger, Joseph P. Emerson, Inorganic Chemistry, 2015 54(5), 2278-2283. Doi:
10.1021/ic502881q
Building reactive copper centers in human carbonic anhydrase II
He Song, Andrew C. Weirtz, Michael P. Hendrich, Edwin A. Lewis, Joseph P. Emerson, Journal of Biological Inorganic
Chemistry, 2013 18(6), 595-598
Calorimetry
Joseph P. Emerson, Vu H. Le, Edwin A. Lewis, eLS (Encyclopedia of Life Sciences), 2012 John Wiley & Sons Ltd, http://www.els.net
Revisiting Zinc Binding in Human Carbonic Anhydrase
He Song, David L. Wilson, Erik R. Farquhar, Edwin A. Lewis, Joseph P. Emerson, Inorganic Chemistry, 2012 51(20),
11098-105.
Exploring Substrate Binding in Homoprotocatechuate 2,3-Dioxygenase using Isothermal Titration Calorimetry
Kate L. Henderson, Vu H. Le, Edwin A. Lewis, Joseph P. Emerson, Journal of Biological Inorganic Chemistry, 2012 17(7), 991-4.
In vivo Self-Hydroxylation of an Fe-Substituted Manganese Dependent Extradiol Dioxygenase
Erik R. Farquhar, Joseph P. Emerson, Kevin D. Koehntop, Milena Trmcic, Mark Reynolds, Lawrence Que, Jr., Journal of
Biological Inorganic Chemistry, 2011, 16(4), 589-97
Human deoxyhypusine hydroxylase, an enzyme that regulates cell growth, has a nonheme diiron active site that binds O2.
Van V. Vu, Joseph P. Emerson, Marlène Martinho, Yeon S. Kim, Eckard Munck, Myung H. Park, Lawrence Que, Jr.,
Proceedings of the National Academy of Sciences, U.S.A.; 2009, 106(35), 14814-9.
Electron Paramagnetic Resonance Detection of Intermediates in the Enzymatic Cycle of an Extradiol Dioxygenase.
William A. Gunderson, Anna I. Zatsman, Joseph P. Emerson, Erik R. Farquhar, Lawrence Que Jr., John D. Lipscomb, Michael P. Hendrich, Journal of the American Chemical Society; 2008, 130, 14465–7
Synthesis, X-Ray Crystallographic Characterization, and Electronic Structure Studies of a Di-Azide Iron(III) Complex: Implications for the Azide Adducts of Iron(III) Superoxide Dismutase
Laurie E. Grove, Jason K. Hallman, Joseph P. Emerson, Jason A. Halfen, Thomas C. Brunold, Inorganic Chemistry; 2008, 47, 5762-74
Swapping Metals in Fe- and Mn-Dependent Dioxygenases. Evidence for Oxygen Activation Without a Change in Metal Redox State.
Joseph P. Emerson, Elena G. Kovaleva, Erik R. Farquhar, John D. Lipscomb, d Lawrence Que, Jr., Proceedings of the
National Academy of Sciences, U.S.A.; 2008, 105, 7347-52
Reaction of Desulfovibrio vulgaris Two-Iron Superoxide Reductase with Superoxide: Insights from Stopped-flow Spectrophotometry.
Victor W. Huang, Joseph P. Emerson, Donald M. Kurtz, Jr., Biochemistry; 2007, 46, 11342 – 51
Structural “Snap-Shots” along Reaction Pathway of Non-heme Iron Enzymes
Joseph P. Emerson, Erik R. Farquhar, Lawrence Que, Jr., Angewandte Chemie International Edition; 2007, 46; 8553 – 6
“Schnappschüsse” von Strukturen entlang der Reaktionswege von Nicht‐Häm‐Eisenenzymen.
Joseph P. Emerson, Erik R. Farquhar, Lawrence Que, Jr., Angewandte Chemie; 2007, 119 (45), 8705-8708
Post-translational self-hydroxylation: a probe for oxygen activation mechanisms in non-heme iron enzymes
Erik R. Farquhar, Kevin D. Koehntop, Joseph P. Emerson, Lawrence Que, Jr., Biochemical and Biophysical Research
Communications; 2005, 338; 230 – 9
The Role of Histidine 200 in MndD, the Mn(II)-dependent 3,4-Dihydroyphenylacetate 2,3-Dioxygenase from Arthrobacter globiformis CM-2 from Site-Directed Mutagenesis Studies.
Joseph P. Emerson, Michelle L. Wagner, Mark F. Reynolds, Lawrence Que, Jr. Michael J. Sadowsky, Lawrence P. Wackett, Journal of Biological Inorganic Chemistry; 2005, 10; 751-760
The 2-His-1-Carboxylate Facial Triad: A Versatile Platform for Dioxygen Activation at Mononuclear Nonheme Iron(II)
Enzymes.
Kevin D. Koehntop, Joseph P. Emerson, Lawrence Que, Jr., Journal of Biological Inorganic Chemistry; 2005, 10(2); 87-93
Iron Enzymes with Mononuclear Nonheme Active Sites.
Joseph P. Emerson, Mark P. Mehn, Lawrence Que, Jr., Encyclopedia of Inorganic Chemistry II; John Wiley & Sons, Inc.; 2005
Kinetics of the Superoxide Reductase Catalytic Cycle.
Joseph P. Emerson, Eric D. Coulter, Robert S. Phillips, Donald M. Kurtz, Jr., Journal of Biological Chemistry; 2003; 278(41); 39662-8
Spectroscopic Characterization of the [Fe(NHis)4(SCys)] site in 2Fe-Superoxide Reductase for Desulfovibrio vulgaris
Michael D. Clay, Joseph P. Emerson, Eric D. Coulter, Donald M. Kurtz, Jr., Michael K. Johnson, Journal of Biological Inorganic Chemistry, 2003; 8; 671-82
An Engineered Two-Iron Superoxide Reductase Lacking the [Fe(SCys)4] Site Retains its Catalytic Properties in vitro and in vivo.
Joseph P. Emerson, Diane E. Cabelli, Donald M. Kurtz, Jr., Proceedings of the National Academy of Sciences, U.S.A.; 2003; 100, 3802-7
Kinetics and Mechanism of Superoxide Reduction by Two-Iron Superoxide Reductase from Desulfovibrio vulgaris.
Joseph P. Emerson, Eric D. Coulter, Diane E. Cabelli, Robert S. Phillips, Donald M. Kurtz, Jr., Biochemistry; 2002; 41(13); 4348-57
Superoxide Reactivity of Rubredoxin Oxidoreductase (Desulfoferrodoxin) from Desulfovibrio vulgaris: A Pulse Radiolysis
Study.
Eric D. Coulter, Joseph P. Emerson, Donald M. Kurtz, Jr., Diane E. Cabelli, Journal of the American Chemical Society; 2000; 122(46); 11555-6. DOI: 10.1021/ja005583r
Remarkably Efficient Olefin Aziridination Mediated by a New Copper(II) Complex
Jason A Halfen, Jason K. Hallman, John A. Shultz, Joseph P. Emerson, Organometallics; 1999; 18(26); 5435-7. DOI: 10.1021/om9908579